| CAS NO: | 161814-49-9 |
| 规格: | ≥98% |
| 包装 | 价格(元) |
| 5mg | 电议 |
| 10mg | 电议 |
| 25mg | 电议 |
| 50mg | 电议 |
| 100mg | 电议 |
| 250mg | 电议 |
| 500mg | 电议 |
| Molecular Weight (MW) | 505.63 |
|---|---|
| Formula | C25H35N3O6S |
| CAS No. | 161814-49-9 (Amprenavir); |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility (In vitro) | DMSO: 100 mg/mL (197.8 mM) |
| Water: <1 mg/mL | |
| Ethanol: 16 mg/mL (31.6 mM) | |
| Other info | Chemical Name: [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate InChi Key: YMARZQAQMVYCKC-UHFFFAOYSA-N InChi Code: InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30) SMILES Code: CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2CCOC2)O)S(=O)(=O)C3=CC=C(C=C3)N |
| Synonyms | 141W94, VX-478, KVX-478; VX 478; VX478; Agenerase; Prozei; KVX 478; KVX478; Amprenavir; |
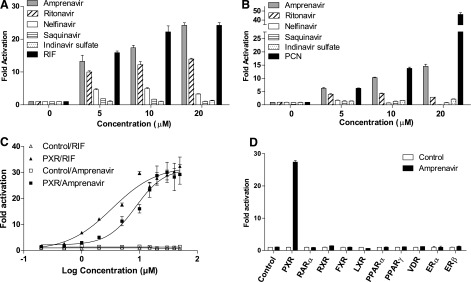
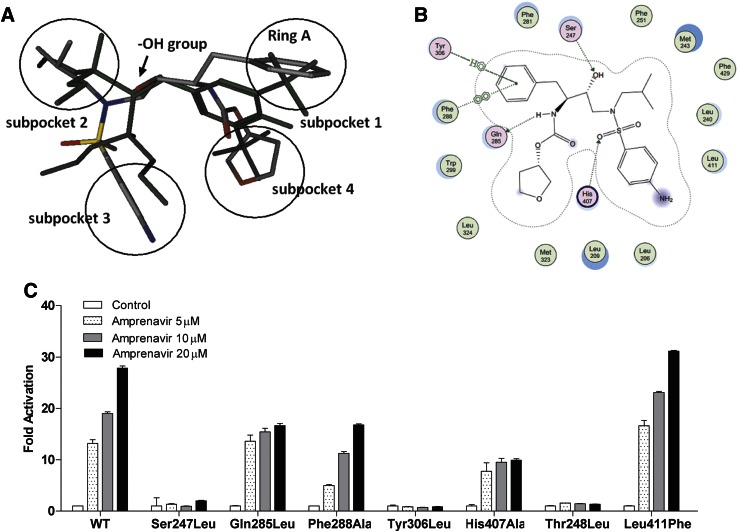
| In Vitro | In vitro activity: Amprenavir promotes the specific interactions between the nuclear receptor pregnane X receptor (PXR) and the coactivators SRC-1 and PBP. Amprenavir is docked into the high-resolution crystal structure of human PXR in complex with SR12813. Amprenavir occupies all four subpockets, and its hydroxyl group forms a hydrogen bond with Ser247, which is located in the connection region of PXR, to help to position the drug in the optimal orientation inside the receptor. Amprenavir forms direct contacts with one residue on αAF of the PXR activation function-2 (AF-2) surface, Phe429, which may stabilize the active AF-2 conformation of the receptor and contribute to the agonist activity of amprenavir on PXR. Amprenavir induces the expression of bona fide PXR target genes involved in phase I (CYP3A4), phase II (UGT1A1), and phase III (MDR1) metabolism in both HepaRG cells and LS180 cells. Cell Assay: Amprenavir induced PXR target gene expression in both HepaRG hepatoma cells and LS180 intestinal cells. |
|---|---|
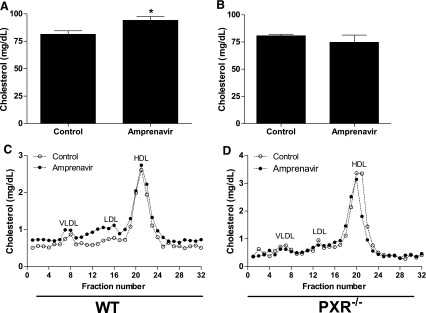
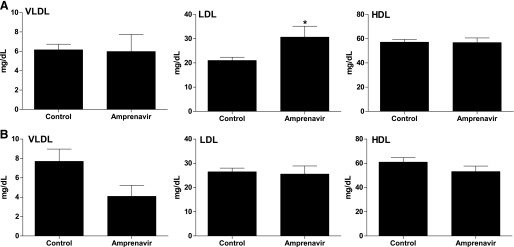
| In Vivo | Amprenavir increases atherogenic LDL cholesterol fractions in WT mice, but not in PXR–/– mice. Amprenavir stimulates expression of known PXR target genes, including CYP3A11, glutathione transferase A1, and MDR1a, in the intestine of WT mice but not in PXR–/– mice. Amprenavir-mediated PXR activation stimulates the expression of both LipF and LipA in the intestine of WT mice, but not in PXR–/– mice, indicating a possible role of intestinal PXR in mediating dietary lipid breakdown and absorption in mammals. |
| Animal model | WT and PXR-/- mice |
| Formulation & Dosage | 10 mg/kg; p.o. |
| References | Mol Pharmacol. 2013 Jun;83(6):1190-9. |
|
|
|  |
 |  |  |
