| CAS NO: | 668270-12-0 |
| 规格: | ≥98% |
| 包装 | 价格(元) |
| 250mg | 电议 |
| 500mg | 电议 |
| 1g | 电议 |
| 2g | 电议 |
| 5g | 电议 |
| Molecular Weight (MW) | 472.54 |
|---|---|
| Formula | C25H28N8O2 |
| CAS No. | 668270-12-0; 1198772-26-7 (Linagliptin mixture with metformin) |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility (In vitro) | DMSO: 17 mg/mL (36.0 mM) |
| Water:<1 mg/mL | |
| Ethanol: 1 mg/mL (2.1 mM) | |
| Solubility (In vivo) | 0.5% hydroxyethyl cellulose: 30 mg/mL |
| Synonyms | Linagliptin; BI-1356; BI1356; BI 1356; trade names: Tradjenta, Trajenta Chemical Name: 8-[(3R)-3-aminopiperidin-1-yl]-7-(but-2-yn-1-yl)-3- methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1H-purine-2,6-dione InChi Key: LTXREWYXXSTFRX-QGZVFWFLSA-N InChi Code: InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 SMILES Code: O=C(N1CC2=NC(C)=C3C=CC=CC3=N2)N(C)C4=C(N(CC#CC)C(N5C[C@H](N)CCC5)=N4)C1=O |
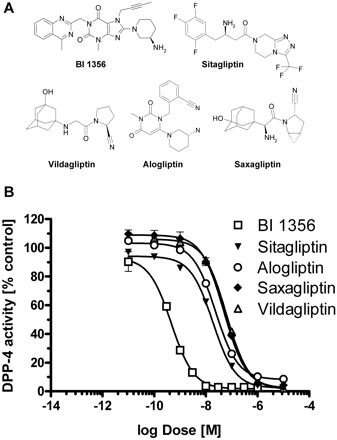
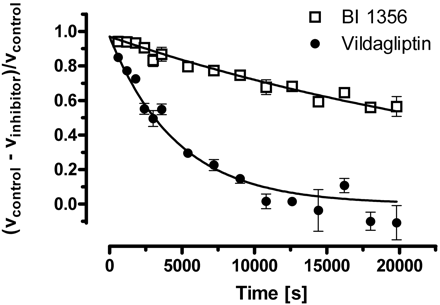
| In Vitro | In vitro activity: Linagliptin shows a potent inhibition effect against DPP-4 in vitro and a low affinity for hERG channel and M1 receptor (IC50 295 nM). Linagliptin acts as a competitive inhibitor with a Ki of 1 nM, and also shows 10,000-fold more selectivity for DPP-4 than DPP-8, DPP-9, amino-peptidases N and P, prolyloligopeptidase, trypsin, plasmin, and thrombin, and 90-fold more selectivity than fibroblast activation protein in vitro. Kinase Assay: EDTA plasma (20 μL) is diluted with 30 μL of DPP-4 assay buffer (100 mM Tris and 100 mM NaCl, adjusted to pH 7.8 with HCl) and mixed with 50 μL of H-Ala-Pro-7-amido-4-trifluoromethylcoumarin. The 200 mM stock solution in dimethylformamide is diluted 1:1000 with water to yield a final concentration of 100 μM. The plate is incubated at room temperature for 10 min, and fluorescence in the wells is determined by using a Victor 1420 Multilabel Counter at an excitation wavelength of 405 nm and an emission wavelength of 535 nm. For the detection of DPP-4 activity in wound lysates, 100 μg of protein from the respective wound lysates are used instead of 20 μL of plasma. Active GLP-1 is also detected from 100 μg of respective wound tissue samples and analyzed by using the Mouse/Rat Total Active GLP-1 Assay Kit. Cell Assay: A total of 4.0×107 keratinocytes per well are seeded into 24-well plates. After reaching 50% confluence, cells are starved for 24 h with DMEM. Proliferation of cells is assessed by using 1 μCi/mL of [3H]methyl-thymidine in DMEM in the presence of 10% fetal bovine serum and increasing concentrations of linagliptin (3, 30, 300, or 600 nM) for 24 h. Cells are then washed twice with phosphate-buffered saline and incubated in 5% trichloroacetic acid at 4°C for 30 min, and the DNA is solubilized in 0.5mol/LNaOH for 30 min at 37°C. Finally, [3H]thymidine incorporation is determined. |
|---|---|
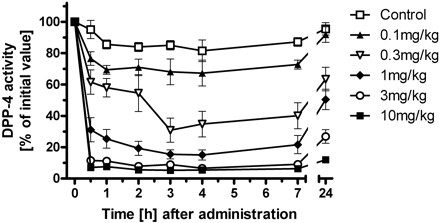
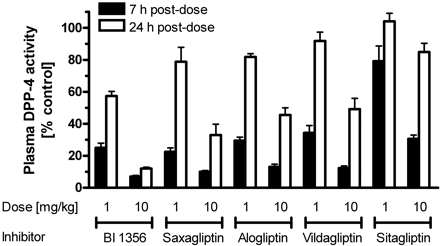
| In Vivo | In male Wistar rats, Beagle dogs, and Rhesus monkeys, Linagliptin shows a highly efficacious, long-lasting, and potent inhibitory activity against DPP-4 by more than 70% inhibition for all three species after oral administration of 1 mg/kg. Oral administration of Linagliptin to db/db mice 45 min before an oral glucose tolerance test reduces plasma glucose excursion in a dose-dependent manner from 0.1 mg/kg (15% inhibition) to 1 mg/kg (66% inhibition). By inhibiting DPP-4 activity, Linagliptin reduces the expression of the proinflammatory markers cyclooxygenase-2 and macrophage inflammatory protein-2, and enhances the formation of myofibroblasts in healing wounds from ob/ob mice. |
| Animal model | Male db/db mice |
| Formulation & Dosage | Dissolved in 0.1 N HCl and subsequently diluted with a 0.5% aqueous hydroxyethylcellulose solution (final HCl concentration 3 mM); 1 mg/kg; p.o. administration |
| References | J Med Chem. 2007 Dec 27;50(26):6450-3; J Pharmacol Exp Ther. 2008 Apr;325(1):175-82. |
 |  |  |
 |  |  |
